How Mycotoxins Affect the Gut
Having spoken to a large number of practitioners about mold cases, I can say for certain that gut issues are one of the most common dilemmas experienced by mold-exposed patients. So today, I thought we’d talk about how exposure to mold can lead to multiple gut-related complications. I’ve recommended mycotoxin testing to hundreds of doctors who were perplexed by their patients’ symptoms; over the years, I’ve begun to notice certain “red flags” on test results that hint at a mycotoxin-related illness.
Three signs that often grabbed my attention were glutathione availability in the body, mitochondrial stress, and gut markers. In this article, we’ll touch on several different gut maladies that may arise in response to toxic mold, including how mycotoxin exposure influences microbiota, immune system function, and gut permeability (with regards to nutrient absorption).
In the last two decades, the link between mold/mycotoxin exposure and chronic illnesses has become more and more evident. Patients can come into contact with mycotoxins from a variety of sources, but the two most common culprits are water-damaged buildings (which actually account for a majority of mycotoxin exposure cases) and food. Now, in the United States, the food industry is heavily monitored by the USDA, which helps prevent folks from being exposed to food-borne mold (1).
However, in developing nations that do not have the resources for extensive monitoring, food can be a significant risk (2). Regardless of the origin point, though, mycotoxins typically enter a host’s body through either the mouth (via breathing or ingestion) or the skin. Mycotoxins then make their way to the blood stream, where they’re filtered out of by the liver, transferred into the gallbladder, and eventually moved into the small intestine. Finally, the mycotoxins are reabsorbed back into the blood stream through the bile acid transporters (3). This continuous recirculation through the intestine amplifies the damage that the toxins do to their host.
The microbiota in our bodies are present in multiple different areas, but the greatest mass exists inside the gastrointestinal tract. The gastrointestinal tract—that is, the “gut” or “bowel”—is made up of multiple winding segments, including the small intestine and the large intestine (AKA the colon), and it extends all the way from the stomach to the anus. The gastrointestinal tract is most well-known for the role it plays in breaking down nutrients into smaller elements that the body can utilize for energy production and growth, but that’s far from its only job: intestines are also critically important for metabolism regulation, immune health, and overseeing the body’s water balance (4).
These functions are made possible through the efforts of the microbiota—a delicate system so complex that it can almost be regarded as its own separate organ living in symbiosis with the gut (5)! Gut microbiota is comprised of many different types of organisms, including bacteria, fungi, and viruses. And because gut microbiota modulate a variety of molecules via metabolization and conjugation (6), it only makes sense that having a diversity of gut bacteria has been linked to better health outcomes (7).
One of the many problems caused by mycotoxins is the disruption of the microbiota homeostasis. One study demonstrated that incubating Ochratoxin A (OTA) with different microbiota caused all of the Lactobacillus reuteri to disappear (8). This is detrimental because Lactobacillus reuteri has anti-inflammatory properties, immunomodulation properties, and can help prevent diarrhea (9, 10). Aflatoxin also decreases the phylogenetic diversity of the microbiota (11)—a reduction that can lead to changes in hormone, metabolism, and immunity modulation.
Mycotoxins have a dramatic effect on the immune system, and this, in turn, has a significant effect on the gut. Multiple mycotoxins cause an inhibition of the immune system, which allows other pathogens to invade. OTA causes an inhibition of the proliferation of peripheral T and B lymphocytes. It also stops the production of interleukin 2 and its receptors (12). Additionally, OTA blocks the production of interferon in the body (13). Animal studies have provided other clues on how mycotoxins affect the body; for example, in dogs, OTA decreases the germinal center of the spleen and lymph nodes and decreases hematopoietic stem cells, which causes a decrease in total white blood cells (14).
Meanwhile, in mice, OTA causes a reduction in mature CD4+ cells (15). All of these changes lead to a major decrease in the body’s ability to fight off infection. Mold-exposed patients’ weakened immune systems predispose them to develop enteric diseases (diseases of the intestine). These types of infections can manifest as diarrhea, depression, inflammation, metabolism trouble, abdominal pain, and as other complications (16). Multiple types of pathogens can also invade the body, including Clostridium (17), E. coli (18), Salmonella (19), and Candida (20).
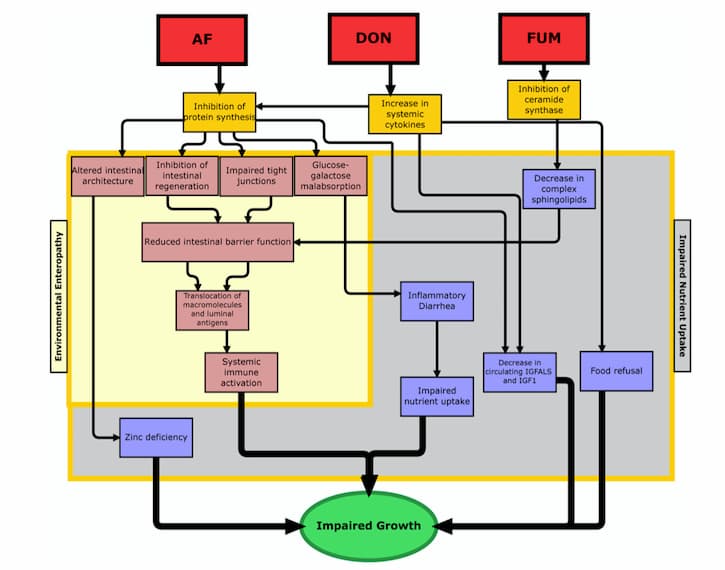
Because the gastrointestinal tract is of such critical importance for many different functions in the body, when it gets damaged by mycotoxins, there can be quite a few consequences. One of the most critical types of impairment that might occur is increased intestinal permeability, which can lead to increased inflammation, loss of nutritional absorption, and translocation of bacteria (21, 22). While the jury’s still out on how, exactly, mycotoxins cause increased intestinal permeability, experts in the field have a few different idea as to why this happens. One prominent theory suggests that mycotoxins may cause an inhibition of the tight-junction proteins (23). Other proposed causes are induced oxidative stress, enterocyte damage (i.e., injury to the intestinal absorption cells), and disruption in sphingolipid metabolism (24-26). All of these changes, after all, can lead to dramatic deviations in the body, which ultimately result in impaired growth (see figure 1 from Smith et al).
Here’s the bottom line: mycotoxin interaction with the intestines can lead to a wide variety issues. And these issues may lead to increased inflammation, co-infections, metabolism troubles, digestive upset, and nutrient absorption impairment. So, when a patient is exposed to mold and takes part in a detox program…that program MUST include real efforts to fix their gut!
1. S. H. Park, D. Kim, J. Kim, Y. Moon, Effects of Mycotoxins on mucosal microbial infection and related pathogenesis. Toxins (Basel) 7, 4484-4502 (2015).
2. L. E. Smith, R. J. Stoltzfus, A. Prendergast, Food chain mycotoxin exposure, gut health, and impaired growth: a conceptual framework. Adv Nutr 3, 526-531 (2012).
3. Z. Zhang et al., A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit Rev Food Sci Nutr 60, 1523-1537 (2020).
4. N. Menche, Biologie Anatomie Physiologie., (Elseveier, Munich, 2012).
5. A. M. O’Hara, F. Shanahan, The gut flora as a forgotten organ. EMBO Rep 7, 688-693 (2006).
6. A. Visconti et al., Interplay between the human gut microbiome and host metabolism. Nat Commun 10, 4505 (2019).
7. A. Mosca, M. Leclerc, J. P. Hugot, Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front Microbiol 7, 455 (2016).
8. M. Ouethrani et al., Metabolic fate of ochratoxin A as a coffee contaminant in a dynamic simulator of the human colon. Food Chem 141, 3291-3300 (2013).
9. L. M. Rocha-Ramirez et al., Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J Immunol Res 2017, 4607491 (2017).
10. K. J. Li et al., Dysbiosis of lower respiratory tract microbiome are associated with inflammation and microbial function variety. Respir Res 20, 272 (2019).
11. J. Wang, L. Tang, T. C. Glenn, J. S. Wang, Aflatoxin B1 Induced Compositional Changes in Gut Microbial Communities of Male F344 Rats. Toxicol Sci 150, 54-63 (2016).
12. T. Lea, K. Steien, F. C. Stormer, Mechanism of ochratoxin A-induced immunosuppression. Mycopathologia 107, 153-159 (1989).
13. A. Pfohl-Leszkowicz et al., New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in human nephropathy and urinary tract tumor. Mol Nutr Food Res 51, 1131-1146 (2007).
14. G. A. Boorman et al., Myelotoxicity and macrophage alteration in mice exposed to ochratoxin A. Toxicol Appl Pharmacol 72, 304-312 (1984).
15. A. Thuvander, E. Funseth, A. Breitholtz-Emanuelsson, I. P. Hallen, A. Oskarsson, Effects of ochratoxin A on the rat immune system after perinatal exposure. Nat Toxins 4, 141-147 (1996).
16. S. G. Cheung et al., Systematic Review of Gut Microbiota and Major Depression. Front Psychiatry 10, 34 (2019).
17. G. Antonissen et al., The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS One 9, e108775 (2014).
18. A. Kumar et al., Pathological changes in broiler chickens fed ochratoxin A and inoculated with Escherichia coli. Avian Pathol 33, 413-417 (2004).
19. J. H. Tai, J. J. Pestka, Impaired murine resistance to Salmonella typhimurium following oral exposure to the trichothecene T-2 toxin. Food Chem Toxicol 26, 691-698 (1988).
20. P. B. Hamilton, J. R. Harris, Interaction of aflatoxicosis with Candida albicans infections and other stresses in chickens. Poult Sci 50, 906-912 (1971).
21. D. I. Campbell, M. Elia, P. G. Lunn, Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J Nutr 133, 1332-1338 (2003).
22. J. R. Kelly et al., Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9, 392 (2015).
23. J. McLaughlin, P. J. Padfield, J. P. Burt, C. A. O’Neill, Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am J Physiol Cell Physiol 287, C1412-1417 (2004).
24. S. Bouhet, E. Le Dorze, S. Peres, J. M. Fairbrother, I. P. Oswald, Mycotoxin fumonisin B1 selectively down-regulates the basal IL-8 expression in pig intestine: in vivo and in vitro studies. Food Chem Toxicol 44, 1768-1773 (2006).
25. Y. M. Abdulrazzaq, N. Osman, A. Ibrahim, Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr 22, 3-9 (2002).26. G. Anderson, M. Seo, M. Berk, A. F. Carvalho, M. Maes, Gut Permeability and Microbiota in Parkinson’s Disease: Role of Depression, Tryptophan Catabolites, Oxidative and Nitrosative Stress and Melatonergic Pathways. Curr Pharm Des22, 6142-6151 (2016).
