Glutathione: Why is it important for detox & how to improve the amount in your body
This month’s blog is focusing on what I consider one of the most important molecules for detoxifying, Glutathione (GSH). Glutathione (GSH) is made in all cells in the body; however, it plays a key role in detoxification in the liver.1 GSH also plays roles in immune function and cellular proliferation (growth of tissue).2 In this article I’m going to touch on three subjects: what is glutathione, what does it do and why is it important for detox, and how to improve the amount in your body?
GSH is a low molecular weight (small) molecule that is called a tripeptide (three amino acids). It is produced by adding cysteine to glutamate and then adding glycine. GSH is used up rapidly in the body. Half of everything that is made is used up every three hours.3 Most of the GSH used for detoxification is produced in the liver and excreted in plasma in the blood stream. In the blood and other cells in the body, GSH works to eliminate compounds that are either from outside the body or metabolites that need to be excreted.
This detoxification function of GSH is extremely important to individuals that have been exposed to environmental toxins such as mycotoxins (mold toxins) and household chemicals. As depicted in Figure 1, GSH binds to these toxins through a strong covalent bond from its reactive sulfur atom.4
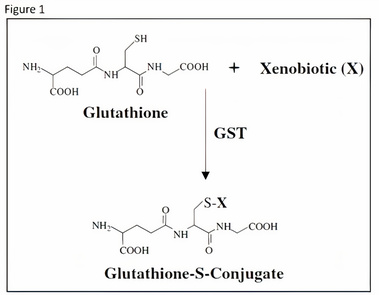
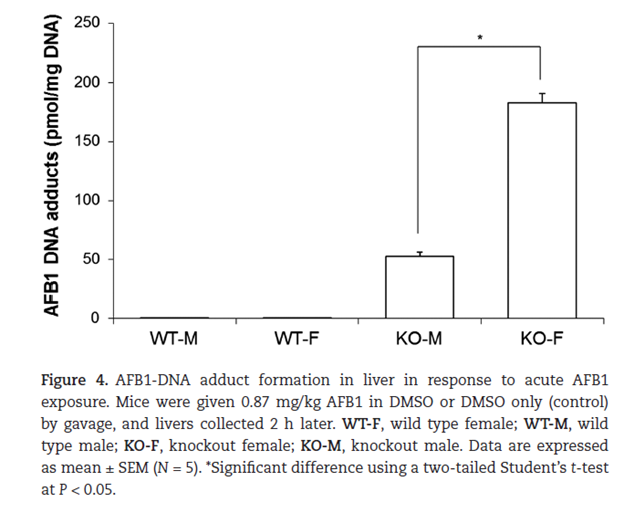
This binding makes the toxic compound more water soluble and assists in the body’s efforts in eliminating the toxin.5 This interaction can occur by itself but occurs at a much faster rate with the assistance of a group of enzymes called the Glutathione-S-Transferases (GST). These enzymes increase the effectiveness of GSH, and patients with mutations in GST can be at greater risk of suffering from environmental toxins. Figure 2 below shows data from an experiment where both wild-type (WT) and GST knockout mice (KO) were both exposed to aflatoxin B1.6 The WT mice had considerably less DNA adducts (molecules that are strongly bonded to DNA that can lead to DNA breaks and mutations) than the KO mice. This can be correlated to a lot of the symptoms that humans suffer from when exposed to mycotoxins. The second interesting figure to observe here is how many more adducts the female KO mice had then the males. This may provide a model why so many more females have symptoms from toxic environments than males. This paper details that the female KO mice had a pronounced reduction in GSTP1 compared to the male KO mice. Also, the male KO mice had an increased CYP1A2 (Part of the P450 enzyme family that are responsible for a large part of detoxification), however this wouldn’t really explain the differences seen in Figure 2.
Increasing the body’s access to glutathione is an important step in detoxifying. However, patients with excessive toxicity should be extremely careful with self-treatment. Mobilization of toxins can lead to complications, and patients with extreme toxicity should seek medical help. I recommend checking our practitioner finder to find a mold specialist near you. Pyroglutamic acid (which is measurable in urine) can help determine if your body is utilizing a lot of GSH. If the measurement is high, then it means that you have toxins in your body, however if the value is too low that could mean that you’ve used up all of your glutathione, or your body doesn’t produce a sufficient amount. There are two reasons a patient may be low on GSH. First, the patient has been exposed to a significant amount of toxins and the body cannot replace the used-up GSH quickly enough. Secondly, genetic differences lead to lower-than-average amount of glutathione production. There are multiple common polymorphisms (genetic differences) that can lead to this outcome. Some are in the methylation pathway and others are in the GSH production pathway. Both can lead to the same result and some practitioners are very skilled in helping patients deal with these hinderances.
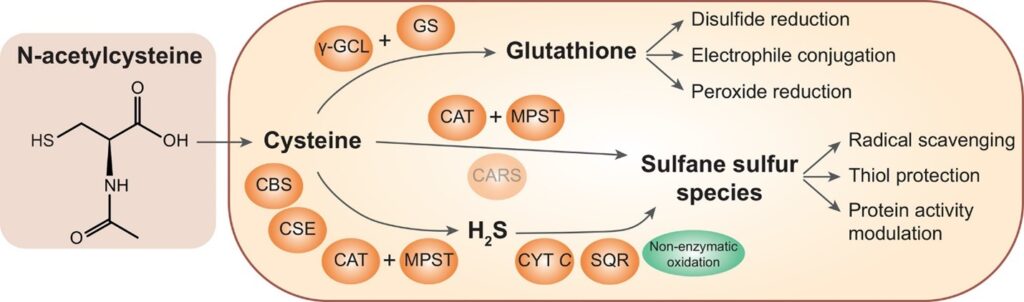
If you are low in glutathione, then there are several ways to improve body reserves. First is to improve upstream pathways such as methylation. The methylation pathway also leads to multiple other pathways (ie. Neurotransmitter production) and adding supplements to assist this pathway can be very tricky. Please don’t try to do this one on your own; you should consult a physician. Second, you can supplement NAC as seen in Figure 3. NAC supplementation can lead to cysteine production and improved production of protective sulfane sulfur species and GSH. NAC is generally non-toxic and excess amounts are normally urinated out. This can be a cost-effective way to help your body detoxify. The third way to improve the body’s reserves of GSH is by skipping the whole production of GSH and supplementing it with the final product. This can be helpful for a lot of patients that have mutations in the GSH production pathway. I have noticed through measurements of NAC in urine that many patients convert NAC to GSH at a low rate; thus, they urinate out a lot of the supplements that they are taking. This leads to a lot of wasted money and lower efficacy of treatment. Utilization of liposomal glutathione can help bypass this problem. As you can see, there are a lot of good products out there to help with this problem.
I hope this article is helpful for someone. We are here to help people get back to their healthy selves.
1. S. C. Lu, Glutathione synthesis. Biochim Biophys Acta 1830, 3143-3153 (2013).
2. H. J. Forman, H. Zhang, A. Rinna, Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med 30, 1-12 (2009).
3. A. Meister, Transport and metabolism of glutathione and gamma-glutamyl amino acids. Biochem Soc Trans 11, 793-794 (1983).
4. D. M. Townsend, K. D. Tew, The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22, 7369-7375 (2003).
5. J. D. Hayes, J. U. Flanagan, I. R. Jowsey, Glutathione transferases. Annu Rev Pharmacol Toxicol 45, 51-88 (2005).
6. D. R. Crawford et al., Characterization of liver injury, oval cell proliferation and cholangiocarcinogenesis in glutathione S-transferase A3 knockout mice. Carcinogenesis 38, 717-727 (2017).
