Overview
Mycophenolic Acid (MPA) and
Mycophenolic Acid Glucuronide (MPAG)
CellCept (Mycophenolate Mofetil) and Myfortic (Mycophenolate Sodium) are therapeutic drugs that are routinely administered to solid organ transplant patients to suppress the immune system and prevent organ rejection. Mycophenolate compounds are metabolized by recipients into Mycophenolic Acid (MPA), the active immunosuppressive compound. MPA is subsequently metabolized into Mycophenolic Acid Glucuronide (MPAG). MPAG is partially converted back to MPA by enterohepatic recirculation.
MPA concentrations are monitored in order to administer the proper dose of medication to the patient. MPAG concentrations are determined to ensure normal metabolism of the drug. Managing the serum levels of both MPA and MPAG will benefit the patient by lowering the risk of organ rejection and toxic side effects.
This test is not available to be purchased by patients. You must have a physician order for RTL to accept and test your sample(s). If you are interested in hearing more about this test, please contact us.
NOT OFFERED OUTSIDE OF THE UNITED STATES

MPA & MPAG
Testing Method
Specimens are analyzed with LC-MS/MS. Ultra-high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) is a powerful analytical tool with superior specificity and sensitivity. This test involves the direct quantification of MPA and MPAG in human serum using a LDT method that has been presented to the American Chemical Society, American Society for Mass Spectrometry, and the Texas Transplantation Society.
Collection Protocol
Timing of specimen collection for Steady State Analysis
Timing of specimen collection for AUC analysis
Note: AUC analysis is at the discretion of the physician and all samples are drawn on the same day.
Note: Please see the collection instructions page included in the kit for more details on collection procedures.
Specimen Requirements
Forms
Turn Around Time (TAT)
Results reported 24-48 hours after the specimen arrives at the laboratory Monday through Sunday.

Clinical Information
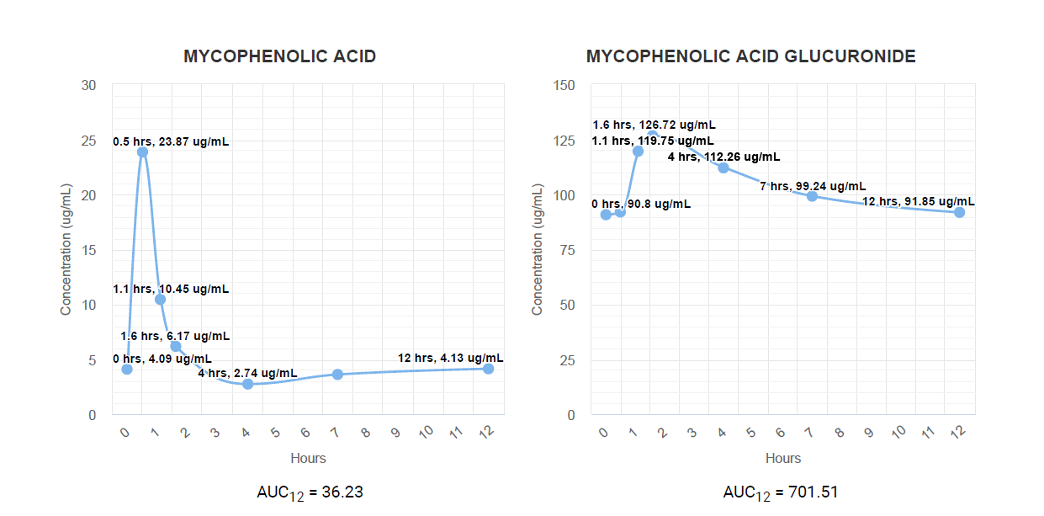
Area Under the Concentration Time Curve Analysis (AUC):
A one-time series of collections consisting of 7 serum specimens over a 12-hour period provides the most comprehensive analysis of the patient’s individual metabolism of Mycophenolic Acid pre- or post- transplantation. MPA and MPAG concentrations are determined for each specimen and compiled to provide an individual pharmacokinetic curve, unique for each patient. The pharmacokinetic curve reveals how patients metabolize MPA and MPAG, allowing physicians to determine the best drug regimen for transplant success.
Monitoring is recommended from immediately after transplant to 3 weeks after therapy is initiated to evaluate dosing adequacy. Additional monitoring is indicated if the MPA level is not in the therapeutic range or if a major change in health status occurs.
Steady State and Follow up testing:
Following this pharmacokinetic (PK) analysis, patients will periodically be tested at the physician’s discretion with one steady state specimen to ensure there are no changes in metabolism, and that the drug is still within the therapeutic range.
The information acquired will help to make treatment decisions about patients to implement personalized medicine.
Interpretation of Results
Reference Ranges
When mycophenolate drugs are given in combination with cyclosporin A, the concentration of MPA is approximately 30-60% lower than when given alone or with tacrolimus or sirolimus.
High dosages of corticosteroids may induce expression of the uridine diphosphate glucuronosyltransferase (UGT), reducing the exposure to MPA.
Other co-medications and co-morbidities can interfere with the absorption, enterohepatic recycling, and metabolism of mycophenolate drugs.
Test Classification
An LC-MS/MS method was developed and validated at RealTime Laboratories, Inc. for the quantification of Mycophenolic Acid (MPA) and Mycophenolic Acid Glucuronide (MPAG) in serum for therapeutic drug monitoring of MPA in solid organ transplant patients. The method was validated following the 2018 FDA Bioanalytical Method Validation Guidance, and is consistent with CLIA requirements. This test is subject to semi-annual proficiency testing by CAP to maintain compliance.